According to the CDC, the majority of patients who were diagnosed with the virus reported using eye drops and artificial tears.
According to the CDC, an early investigation revealed ten distinct brands that may have been connected to the incident. Eyedrops that were manufactured in India and sold in the United States under two different brand names were ultimately removed from store shelves in the months of January and February.
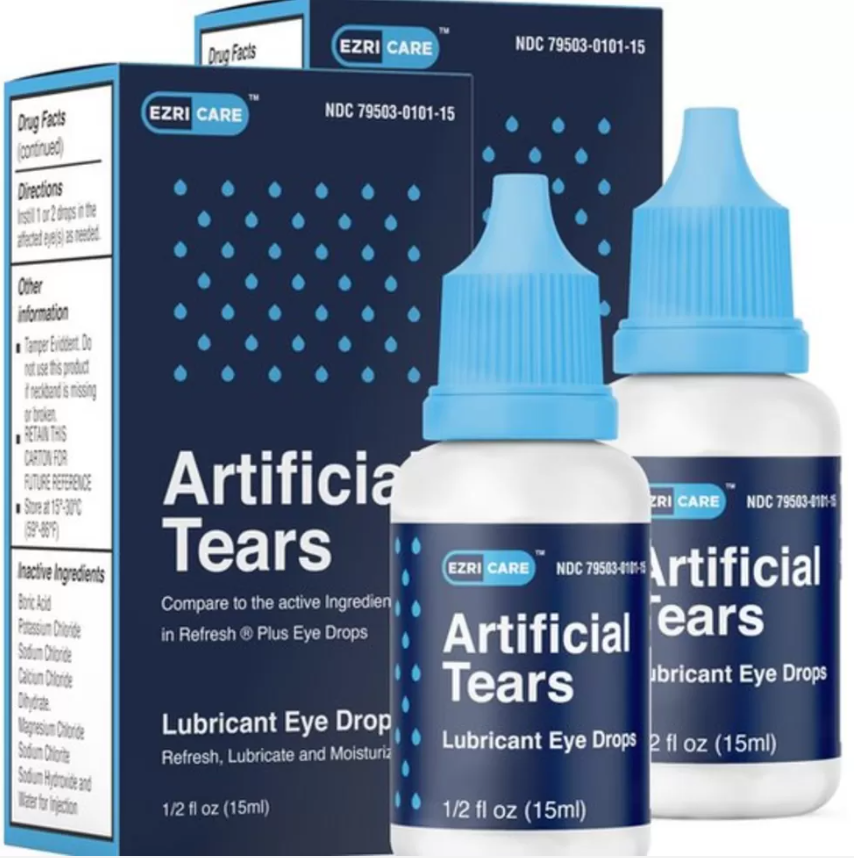
Injuries and blindness have prompted a recall of an eyedrop product in the United States.
The Centers for Disease Control and Prevention (CDC) issued a warning in January advising consumers to cease using EzriCare Artificial Tears and Artificial Tears manufactured by Delsam Pharma. After receiving an official recommendation from the Food and Drug Administration, the next month, the business that owns the brands, Global Pharma, announced a voluntary recall of the products (FDA).
The CDC reported that open bottles that were removed from patients and tested had the germs after the bottles were opened. At this time, unopened bottles are being examined in order to ascertain whether or not the production procedure resulted in the introduction of contamination.
A lady in Florida filed a lawsuit against the pharmaceutical business only the week before last, saying that an infection she got from taking their treatment caused her physicians to have to remove one of her eyes.
The contamination was blamed on the eyedrops’ absence of preservatives, according to a lawyer for the victim.
“It’s very possible that there are a great deal more people who are ignorant that they have infections,”
A spokesman for EzriCare has said that the epidemic has not been clearly connected to any of their products based on the testing that has been done so far.
According to a statement made by a spokesman, “We have been contacting clients to the maximum degree feasible in order to caution them against continuing to use the product.”
“At the same time, we contacted the Food and Drug Administration as well as the CDC, and we made it clear that we are ready to comply with whatever demands they may have of us,”
According to the CDC, anybody who has used the recalled goods and is now experiencing symptoms should get in touch with a medical professional as soon as possible.
The discharge from the affected eye may be yellow, green, or clear, and other symptoms include irritation or soreness, redness, blurred vision, and an increased sensitivity to light.
Following reports from Pharmedica and Apotex that they removed certain eyedrop products from stores on their own will, the Food and Drug Administration (FDA) issued separate recall notifications for certain eyedrop products last week.
According to Statista, a market research company, there were around 117 million people in the United States who used eyedrops and eyewash products in the year 2020.